
In my first year of university my friend Theevan had chosen an elective called “chimie de la santé” which was chemistry focused on medicines. One the practical labs for this course was the synthesis of soap. I had chosen different electives, but I was still able to see the product they had made at the end of the lab.
Seeing and hearing about how easy it was to actually synthesize soap opened up a rabbit hole on soap making that I ended up getting lost in for quite a while. I thought making a small soap selling business would have been a cool side hustle I could start in order to make a little money on the side.
Quick overview

Making soap in the basic sense consists of three steps;
Making lye
Lye is an aqueous solution containing water (H2O) and an alkali such as sodium hydroxide (NaOH). Sodium is considered a strong base, capable of completely disassociating in aqueous solution to form Na+ and OH–. The reaction is exothermic, meaning it gets really fucking hot (it’s also corrosive, so handle with caution. Look up lye attacks if you’re lacking the proper respect).
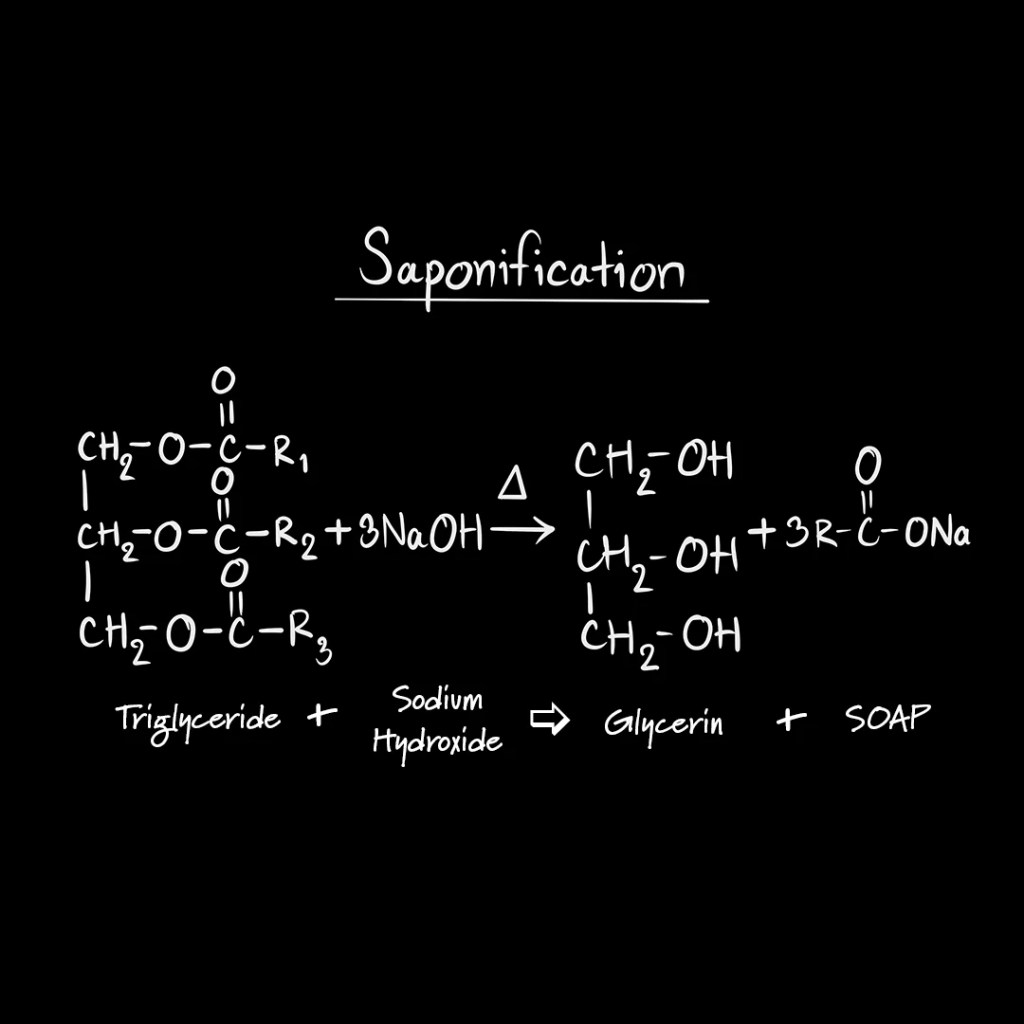
Reacting lye with a fatty acid
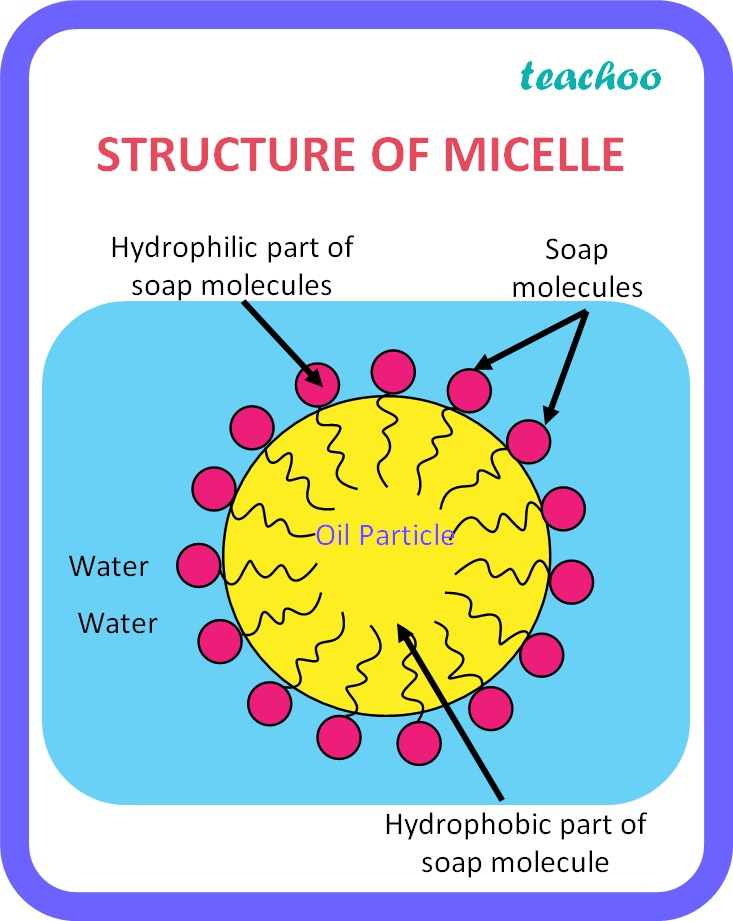
Fats and oils contain triglycerides, which can react with the aqueous alkali in the lye in a reaction called saponification. Saponification put simply is putting in a triglyceride and three strong base molecules to get one glycerol and three fatty salt molecules. These fatty salt molecules contain a hydrophobic and hydrophilic segement, making them amphiphilic. An emergent property of simple amphiphilic molecules is the formation of micelles. For brevity, I will describe micelles as little shopping bags which carry dirty away.
Setting
The final step is just allowing the soap to cure. This allows the saponification reaction to complete while allowing extra water to evaporate. This helps reduce solubility, so the soap can harden and doesn’t disintegrate after one use.
In practice there are several other steps, such as calculating lye ratio, concentration and triglyceride mass. pH testing is done in professional settings to ensure all the lye has been removed, and the soap is safe. Soap making has a large community of people who introduce variations to make different smells with essential oils, or use different fats to attain varying levels of lathering (how bubbly the soap gets), as well as altering the form of the soap between liquid and solid states.
My Personal Experience
I wanted to get a good feel on how oil ratios effected the quality of the soap, so i made a variety of soaps to achieve this.
| Oil/Fat type | A | B | C | D | E |
| Olive(%) | 100 | 0 | 35 | 65 | 50 |
| Coconut(%) | 0 | 100 | 65 | 35 | 50 |
The trends from the samples showed that olive oil allows for the soap to be soft, but a too high of a percent (<65%) leads too a soap that takes an unreasonable time to solidify, around a week compared to coconut oil which sets within a day(complete curing of olive oil takes even longer, around a month and a half compared to a month or so with coconut oil). When it comes to coconut oil, the increase of it corresponded with higher ability to lather, but a higher percent (<65%) leads to a bar that’s very hard, and difficult (but not impossible) to initiate a lather with.
The 50/50 sample was by far the best for lathering, softeness and overall aesthetic. I ended up attepting to create three additional samples with the same ratio, but includes bees wax (ranging from (0.5 to 1.5 %). The desired effect was to create a soap with more structure without increasing hardness. The results were however inconclusive.
that’s about it for this recap, Take care!

